
Call Us
+1-469-428-5508
Our Location
Dallas Texas

+1-469-428-5508
Dallas Texas
AmWiner Raphe Pharma has expertise in developing and making tastemasked products using fluid bed coating, ion exchange, and other technologies. We have successfully developed a taste-masking formula to mask exceedingly unpleasant flavors at particle sizes of less than 40 microns.
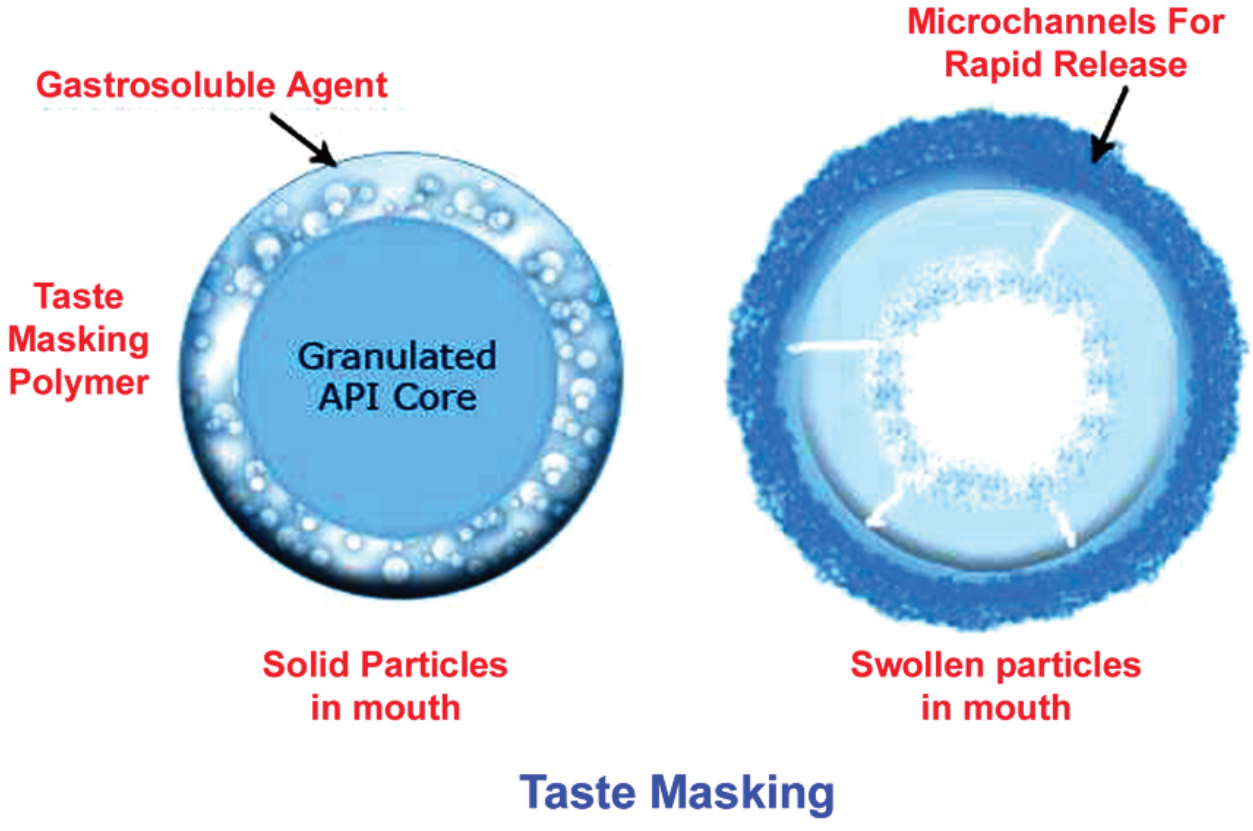
AmWiner Raphe Pharma’s formulations are customized to each unique situation. We begin by identifying the optimal coating material to establish the optimal system for each project. Our highly trained technicians evaluate all formulation options during this phase to create the final product. The system is then designed to fulfil the desired function and coating material restrictions. The form or variant of the core material is evaluated, along with its particle size, cost, and production requirements.
Powder can be pressed into a variety of tablet sizes and shapes using a tooling press. Our multiple blending operation styles allow drug distribution throughout the tablet. We can handle any size of project, from research to commercial manufacturing, with our 3-stations tableting machines.


The mechanism of controlled release is achieved via a matrix or core-shell structure that offers obstacles to the drug’s release. Controlled release of active ingredients is achieved through a matrix or core-shell structure in which the matrix or shell functions as an obstacle. A porosity, solubility, or erosion property of the matrix is used to slowly release the active ingredient through the outer coating at a defined rate.
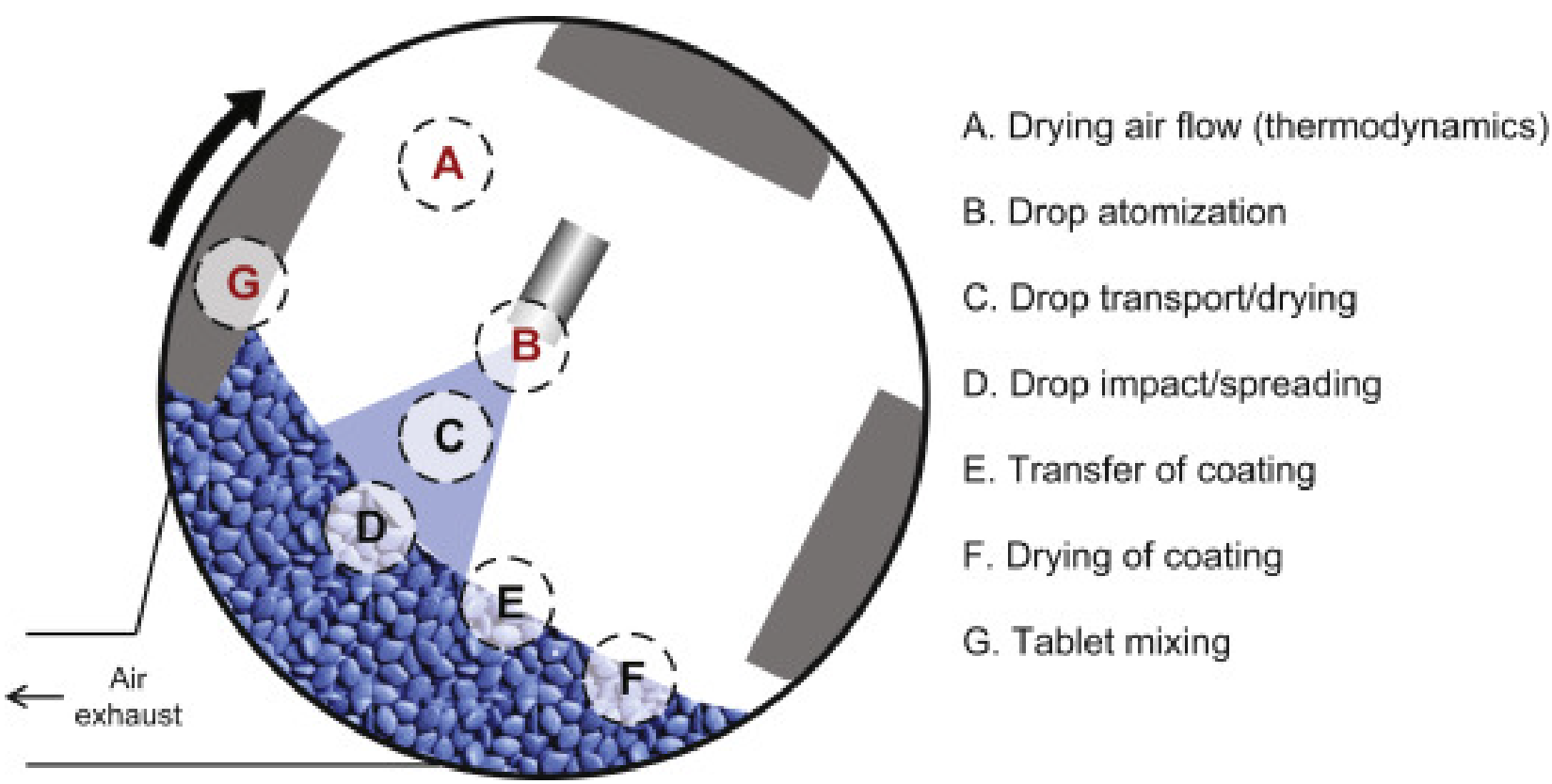
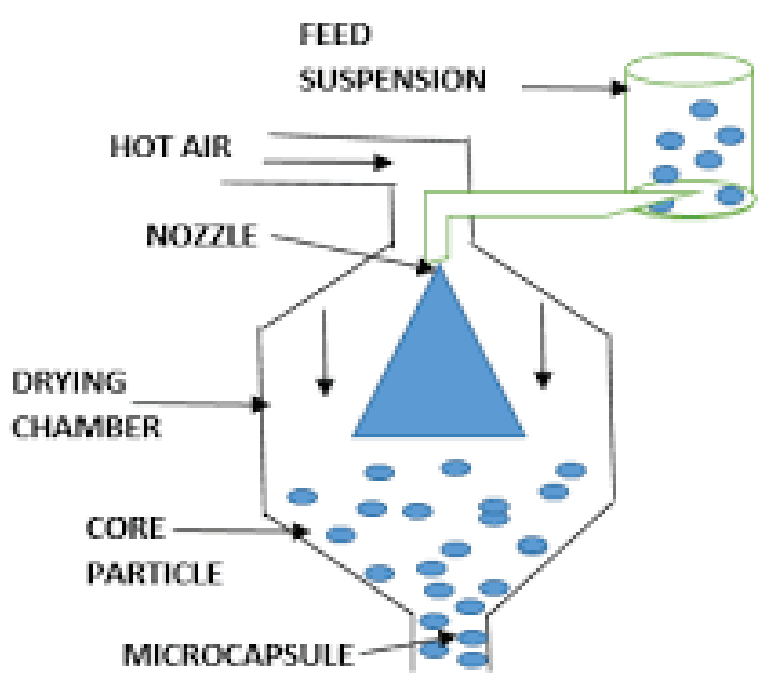
Since its establishment in 2005, AmWiner Raphe Pharmaceutique has been a leader in fluid bed coating technology, which encapsulates tiny particles in a fluidized bed by creating a cyclic air flow in a differential air flow. Our process differs from other coating procedures in that the nozzle is situated at the bottom of the fluidized bed of particles. When a substance is atomized and sprayed throughout the chamber, a cyclic air stream moves the bed of particles upward as it is coated to form a core-shell composition.
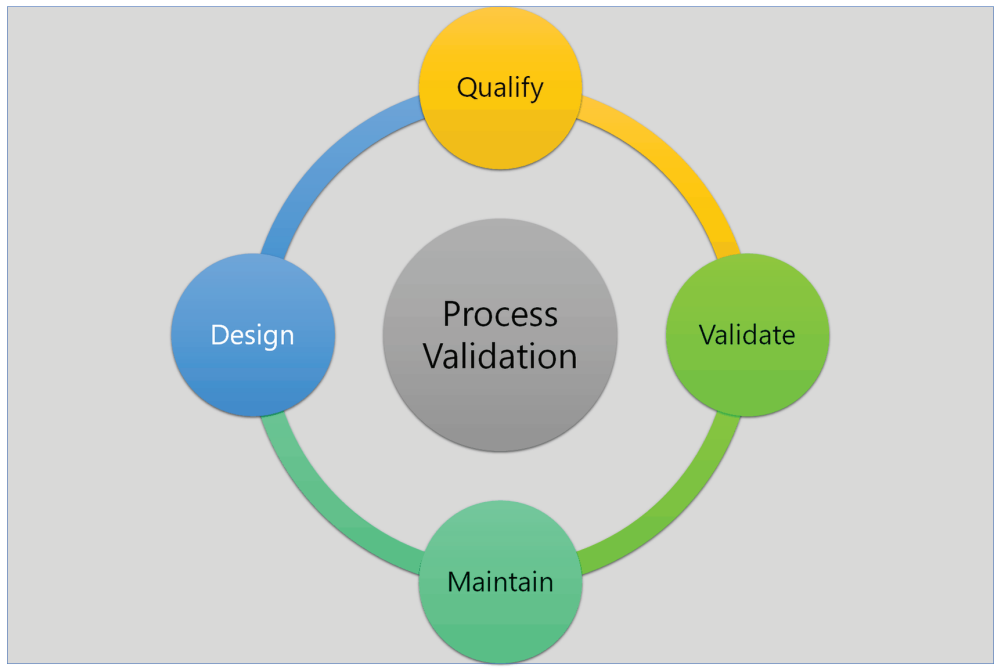
There’s really no product or market in which quality is not important. A final validation report is prepared once all required work has been completed and satisfactory results have been achieved, and the procedure is validated for mass production. Raphe has the workforce and resources to accomplish every phase of process validation, from developing a process validation protocol to compiling the final process validation report. Virtually every product in any market and industry considers quality to be important.