The Challenges in pharmaceutical drug formulation have increased significantly in recent decades, while at the same time the market situation has grown increasing-ly challenging. New chemical entities in the pipeline are facing solubility challenges, with about 80 percent of them reported as having poor solubility. Since formula-tion solubility is a critical prerequisite for good bioavailability and therapeutic efficacy, finding ways to improve solubility without changing the chemical nature of theNCE has never been more important.
In addition, the current industry trends towards regionalization and personalization of treatment approaches necessitate faster, more efficient, and increasingly flexi- ble solutions. As a result, continuous pharmaceutical manufacturing is currently in a prominent field of development, driving the need for tighter process controls and more thorough process and product understanding. An intelligence technology having access to a wide range of suitable excipients is a prerequisite for a successful formulation approach, and this is what we offer to pharmaceutical industries.
This is becoming increasingly important as drivers of innovation in drug develop- ment. It has recently been estimated that most drugs currently in development are in the hands of small biopharmaceutical companies. Formulation is one of the most crucial parts of the drug development process. We must be able to deliver the drug to the body in whatever dosage form necessary that provides the best effect for that medication. Without proper formulation that guarantees solubility and bioavailability, patients’ health and healthcare system will be at tremendous risk. The small pharmaceutical drug companies want to quickly develop formula- tions for clinical trials, at the same time, but they have reduced resources and technology challenges to develop the most cost- effective formulation as quickly as possible, the quicker the medication can make its way to patients, the faster we can impact the worlds healthcare system. AmWiner makes it possible for every biopharmaceutical industry to achieve this either by loaning these technol- ogies or developing the drugs for you at our state of arts facility in Dallas Texas


This is becoming increasingly important as drivers of innovation in drug develop- ment. It has recently been estimated that most drugs currently in development are in the hands of small biopharmaceutical companies. Formulation is one of the most crucial parts of the drug development process. We must be able to deliver the drug to the body in whatever dosage form necessary that provides the best effect for that medication. Without proper formulation that guarantees solubility and bioavailability, patients’ health and healthcare system will be at tremendous risk. The small pharmaceutical drug companies want to quickly develop formula- tions for clinical trials, at the same time, but they have reduced resources and technology challenges to develop the most cost- effective formulation as quickly as possible, the quicker the medication can make its way to patients, the faster we can impact the worlds healthcare system. AmWiner makes it possible for every biopharmaceutical industry to achieve this either by loaning these technol- ogies or developing the drugs for you at our state of arts facility in Dallas Texas
This includes the use of new technical platforms, the use of new formulations or technologies that enhance the actions of known drugs. One example is a new, thermostable polymer for hot-melt extrusion processes that combines stabilization of the solid amorphous form in the tablet and inhibition of drug precipitation in dissolution. Another formulation technology being used is a silica-based drug carrier that features a functionalized particle structure that allows for API adsorption onto its surface and pores, stabilizing the drug in a solu- ble amorphous form. These two methods have not yielded a definitive success that the need for intelligent technology capable of guaranteeing effective formulation will help strengthen and improve our healthcare system and patients’ wellbeing. When faced with formulation questions and challenges, AmWiner is your partner in drug manufacturing, turn to us for assistance, all advise is free.


AmWiner has made a remarkable transformation of pharmaceutical industry in drug development and patient care with the integration of Artificial Intelligence (AI) in medicine development and efficacy testing. The use of AI will revolutionize the way drug products are formulated, dosed, and tested for stability and efficacy. Understanding formulation technology in drug development is a field of artificial intelligence (AI) in which software learns from imputed data to perform a task throughout the drug development and manufacturing process that will increase drug efficiency and effectiveness, decreasing the time and cost required to bring new drugs to market. Giving small pharmaceutical companies the opportunity to be part of the manufacturing process of bringing new drugs with optimum solubility and bioavailability to the market and increasing the supply of the existing ones. These improvements will save lives and reduce suffering by getting drugs to patients in need more quickly and could allow researchers to invest more resources in areas such as rare or orphan. This is one of the areas AmWiner comes in to sup- port every pharmaceutical practice, in getting the work done with highest purity, efficacy and on time.
Utilizing our intelligence technology gives you access to the best and accurate percentage of Active Pharmacological Ingredient/s and expedient percentages to use in any formulation to yield the desired expected results, taking away the human error of miscalculation that contributes to some adverse effects in drug. When con- fronted with a customized medicine, you`re confident that the technology will deter- mine the accurate percentage of the active and expedients needed to use based on the imputed objective you intend to achieve. Even when you add any ingredient more or less of what is required, the AmWiner “AI-PHARMA technology” will indicate which ingredient/s you have in excess and what to do to balance your formulation, expediting the formulation and optimizing the dosages. Through this advanced algo- rithm of the AI-PHARMA technology analyzes the finished product to produce a more accurate certificate of analysis and SDS document, conduct stability product testing, shelf-life test, and efficacy testing.
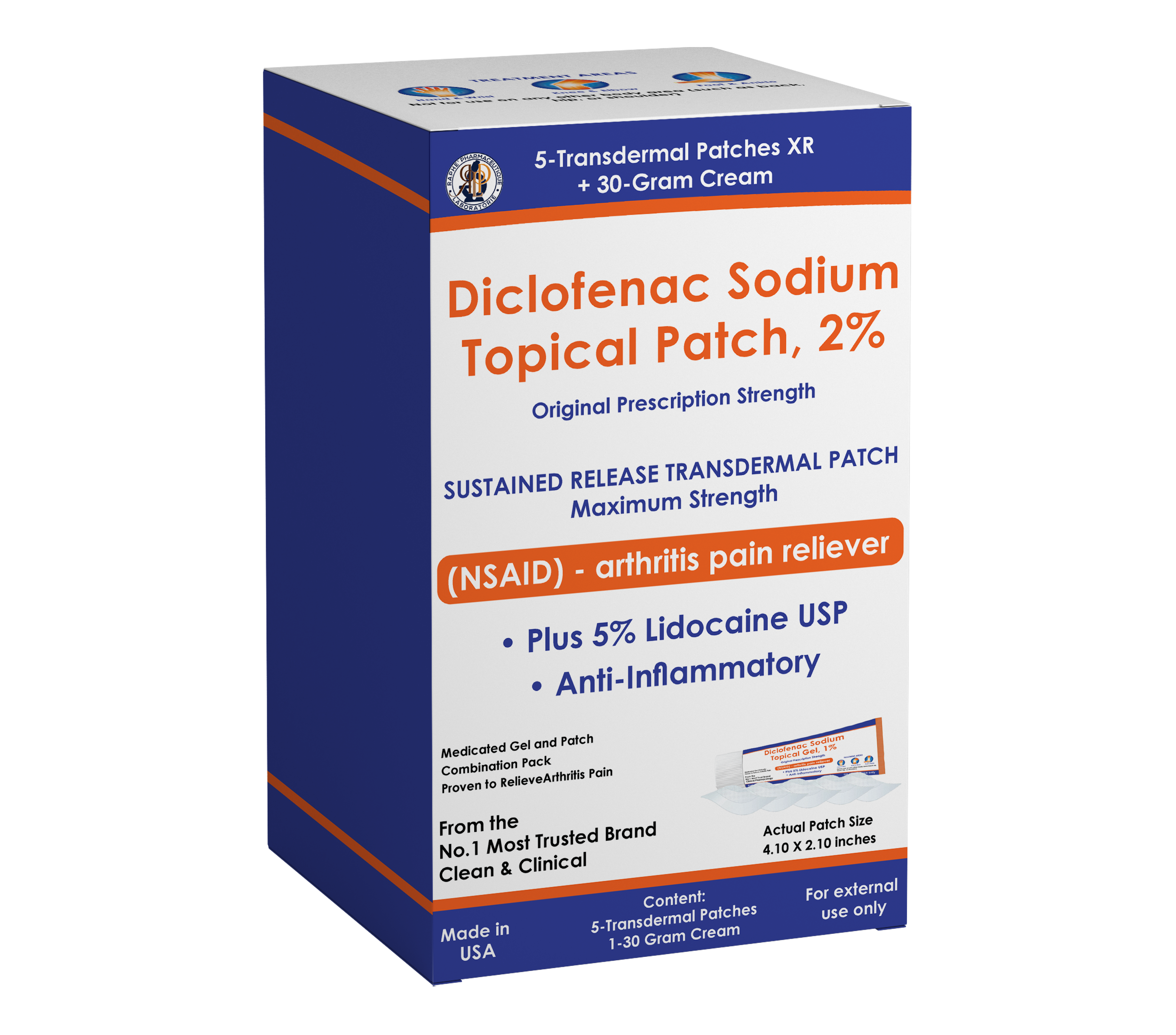

When conducting Quality control testing on topical, liquid or solid dosages such as tablets and capsules as in friability tests, tablet disintegration tests, dissolution tests, and moisture content tests. These tool algorithms give you more access to other parameters in the that are easily forgotten during drug product quality testing. They are powerful engineers in tablet coating when manufacturing XR or enteric-coated pills. It determines the exact percentage of the coating ingredient required and shows the best coating substance suitable for each tablet moiety. You can take the drying and chipping issues of the coating process out of your list of worries because this technology delivers 100% accurate coating technique and guide for your batches.
And dermatological conditions, and ingredient interactions to identify potential compounds, ingredients, and therapeutic interventions necessary for every skincare or bodily concern. This not only accelerates the research and development phase but also enables the creation of highly effective and personalized solutions. Moreover, these AI algorithms will predict the performance of different formulations, leading to the creation of products that offer superior outcomes in addressing various patients’ skin concerns. To maximize the benefits of this technology, biochemists will prioritize data quality and diversity, ensuring that the algorithms collect comprehensive datasets that encompass a wide range of demographics, and dermatological conditions. This inclusive approach to data collection and analysis will foster the creation of effective drug products across diverse populations and demographics, promoting inclusivity and accessibility in healthcare systems. By fostering partnerships between dermatologists, pharmacologists, data scientists, and tech experts, drug product developers can leverage diverse expertise to optimize formulation strategies, dosing protocols, and efficacy testing methodologies. This collaborative approach not only enriches the insights generated by the algorithm but also ensures that drug products are developed with a comprehensive understanding of human biology, therapeutic mechanisms, and patient needs

By analyzing molecular structures and biological pathways, these AI algorithms pinpoint promising treatments for any skincare or bodily issues, thus streamlining the therapeutic process. This approach opens new avenues for developing inno- vative treatments for common skin and body ailments, or customized medicines for HRP, acne, vitiligo, rosacea, pigmentation, eczema, and aging-related condi- tions and enable researchers to assess the safety and efficacy of potential drug products, fostering the creation of products that are not only effective but also safe for prolonged use. In essence, AmWiner`s technology revolutionizes pharma- ceutical drug development by empowering researchers to navigate the complex- ities of human biology and formulation optimization with unprecedented speed and precision. The insights generated by the algorithm will pave the way for the creation of next- generation drug products that cater to diverse health condi- tions, marking a paradigm shift in the field of dermatology and general medicine.
Accurate dosing is paramount to ensure optimal therapeutic outcomes and mini- mize adverse effects. AmWiner Pharmacy formulation AI technology” will play a pivotal role in precision dosing by leveraging data analytics and predictive model- ing to determine the most effective dosage regimens for any drug product. By analyzing patient-specific factors, such as skin type, age, and medical history, the AmWiner AI algorithms can tailor dosing recommendations to individual needs, thereby enhancing the efficacy and safety of treatments. This technology will adapt to real-time changes in patient responses and adjust dosage parameters accordingly. This dynamic dosing approach will enable the healthcare profession- als to fine-tune treatment plans based on evolving patient conditions, ultimately optimizing the therapeutic benefits of the medicines. Additionally, the AI algo- rithms will identify potential drug interactions and contraindications, offering valu- able insights to healthcare providers and patients alike. By harnessing the power of AmWiner Pharmacy formulation AI technology” in medicine dosing, the pharmaceutical industry is poised to deliver personalized treatment regimens that are tailored to everyone’s unique physiological and dermatological profile. This not only maximizes the efficacy of the drug product but also minimizes the risk of adverse reactions, setting a new standard for preci- sion and safety in dermatological care.


Ensuring the stability of any drug product is essential to maintain its efficacy and safety over time. This technology emerges as a game-changer in product stability testing, enabling QC team to assess the impact of various environmental factors, storage conditions, and formulation changes on the shelf-life and performance of their drug products. Through predictive modeling and accelerated testing meth- odologies, the algorithms will predict the degradation kinetics of active ingredi- ents, anticipate potential formulation instabilities, and optimize packaging materi- als to prolong product shelf-life. This intelligence-powered stability testing moiety allows for the rapid identification of formulation modifications that can enhance product stability without compromising efficacy. By simulating different storage scenarios and analyzing real-time data from stability studies, these algorithms will recommend adjustments to formulation parameters, packaging designs, and storage protocols to mitigate degradation risks and extend the lifespan of drug products. This proactive approach to stability testing minimizes product wastage and ensures consistent efficacy throughout the product’s shelf-life, thereby enhancing customer satisfaction and brand reputation. Furthermore, it will facili- tate the early detection of stability issues by flagging potential risks based on predictive analytics and historical data. This proactive monitoring capability will empower drug product manufacturers to preemptively address stability con- cerns, implement corrective measures, and maintain the integrity of their prod- ucts throughout their lifecycle. As a result, the AI-driven stability testing not only streamlines the product development process but also fosters the creation of high- quality drug products that meet stringent stability and performance stan- dards.
Continuous validation and refinement of these intelligence technology models are critical to maintaining the accuracy and reliability of drug development processes. The biochemist shall regularly validate the algorithms against real-world clinical data, patient outcomes, and market feedback to ensure that the insights and recommen- dations offered by AmWiner Raphe Pharmaceutique Laboratory technologies remain aligned with actual patient needs and treatment outcomes. To develop a drug product with highest precision, efficacy and purity or to explore cutting-edge solutions in dermatology, call us at: 1-469-428-5505 or email [email protected]
