Description
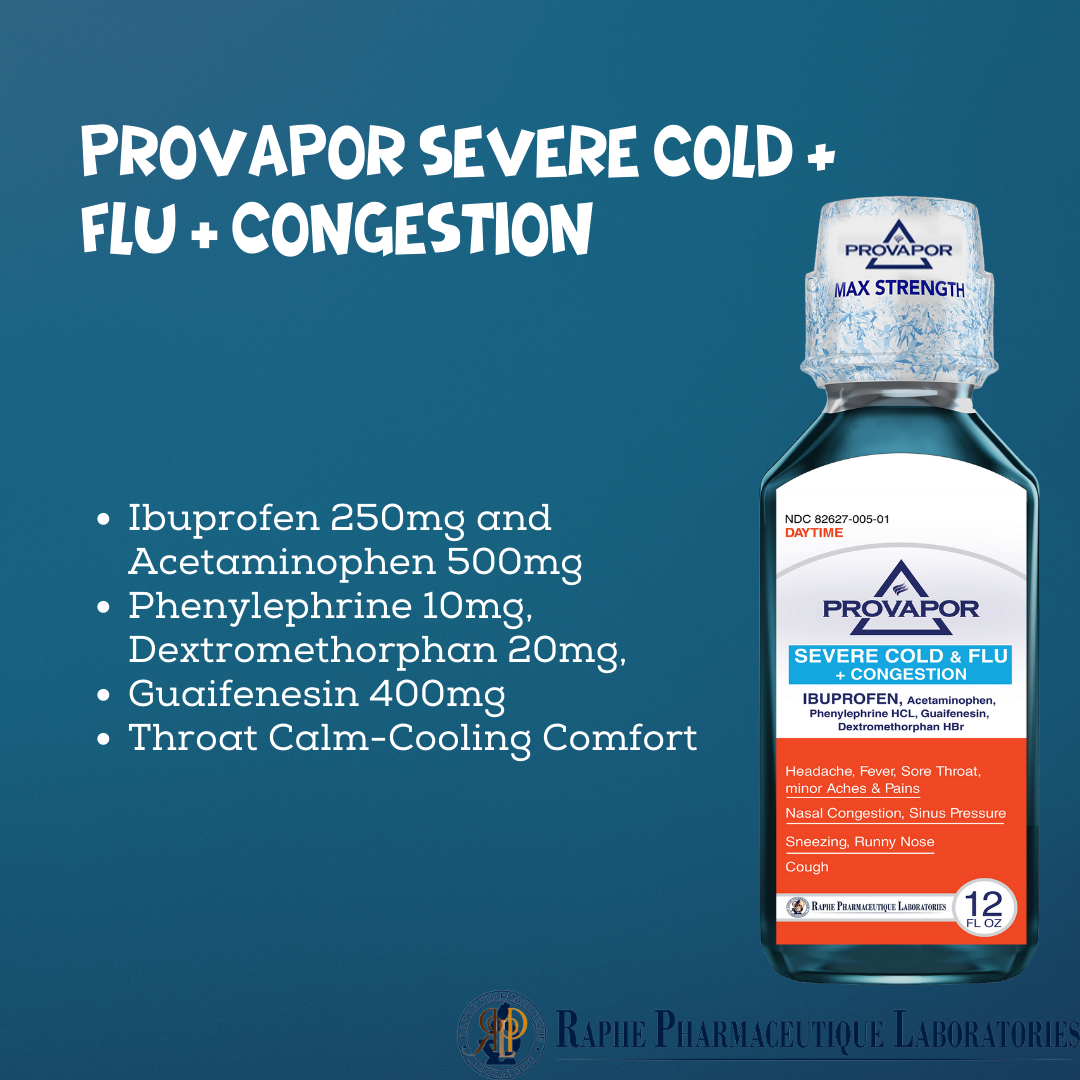
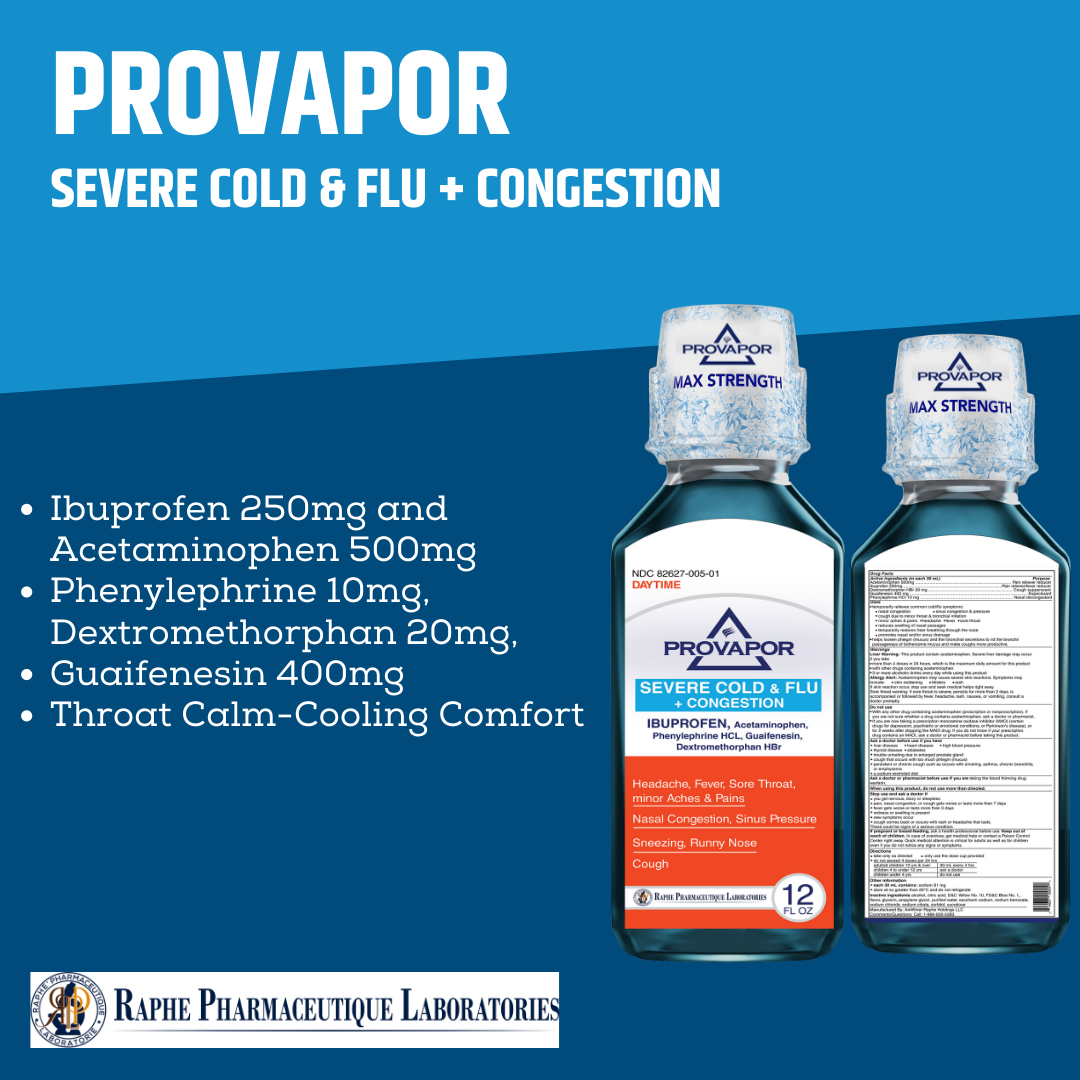
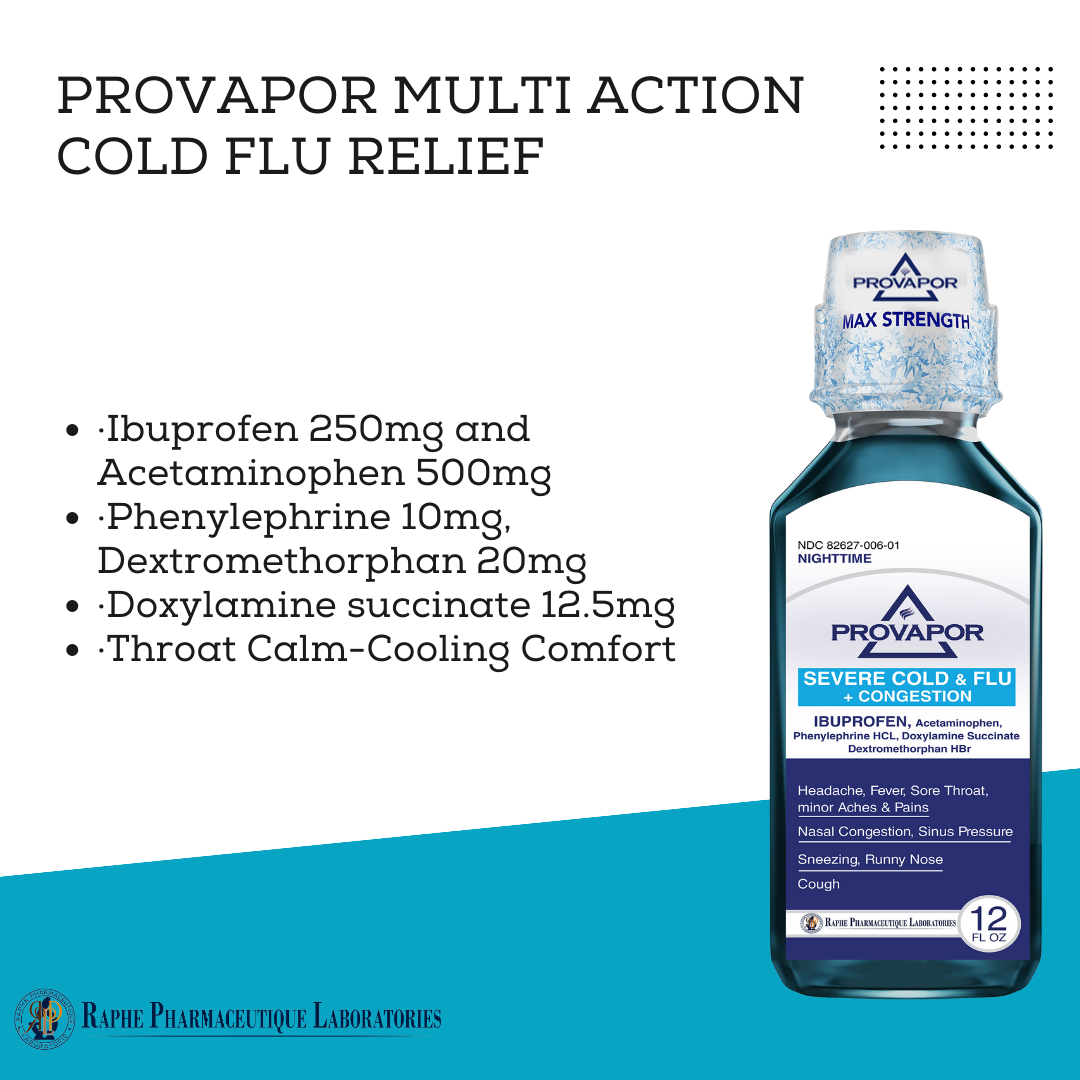
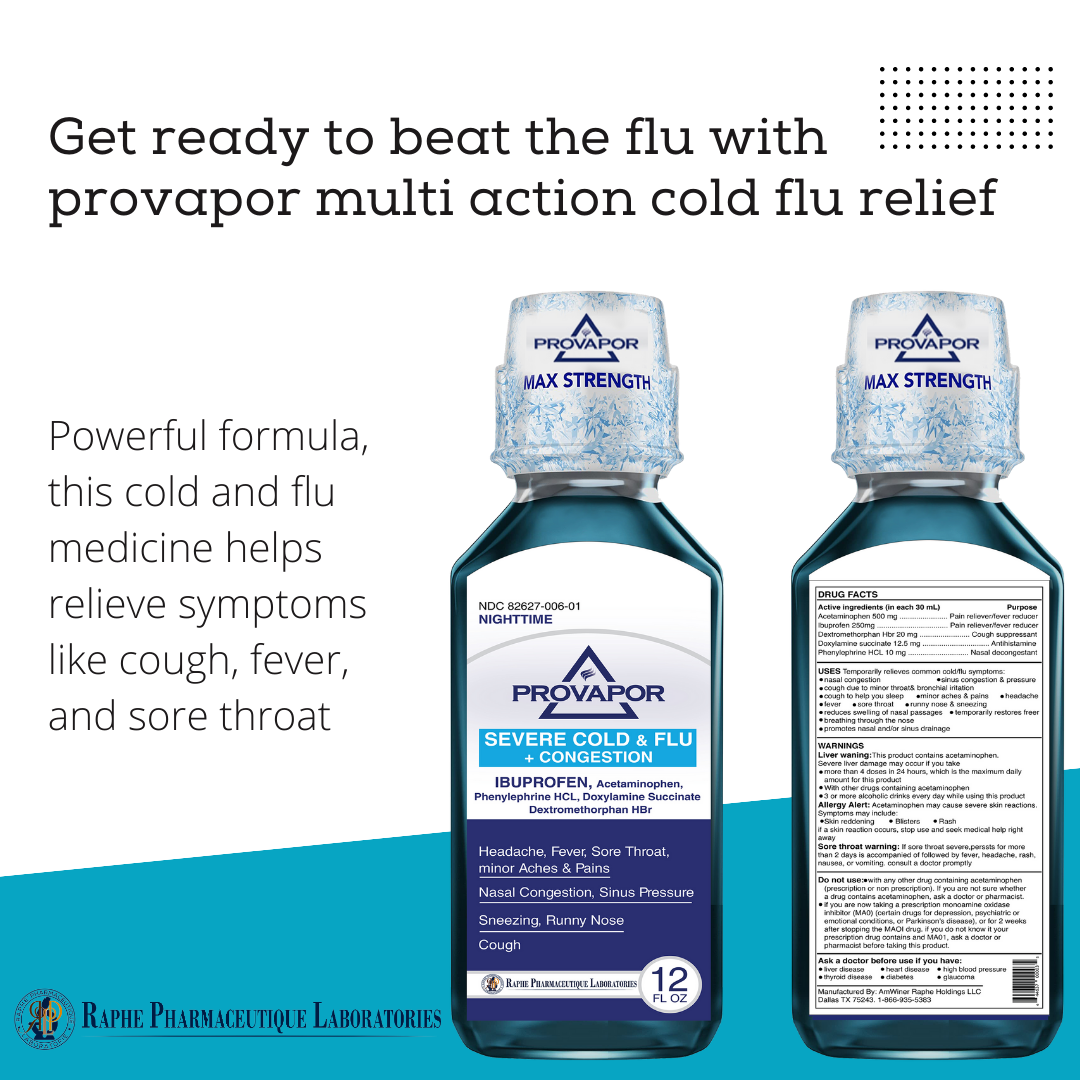
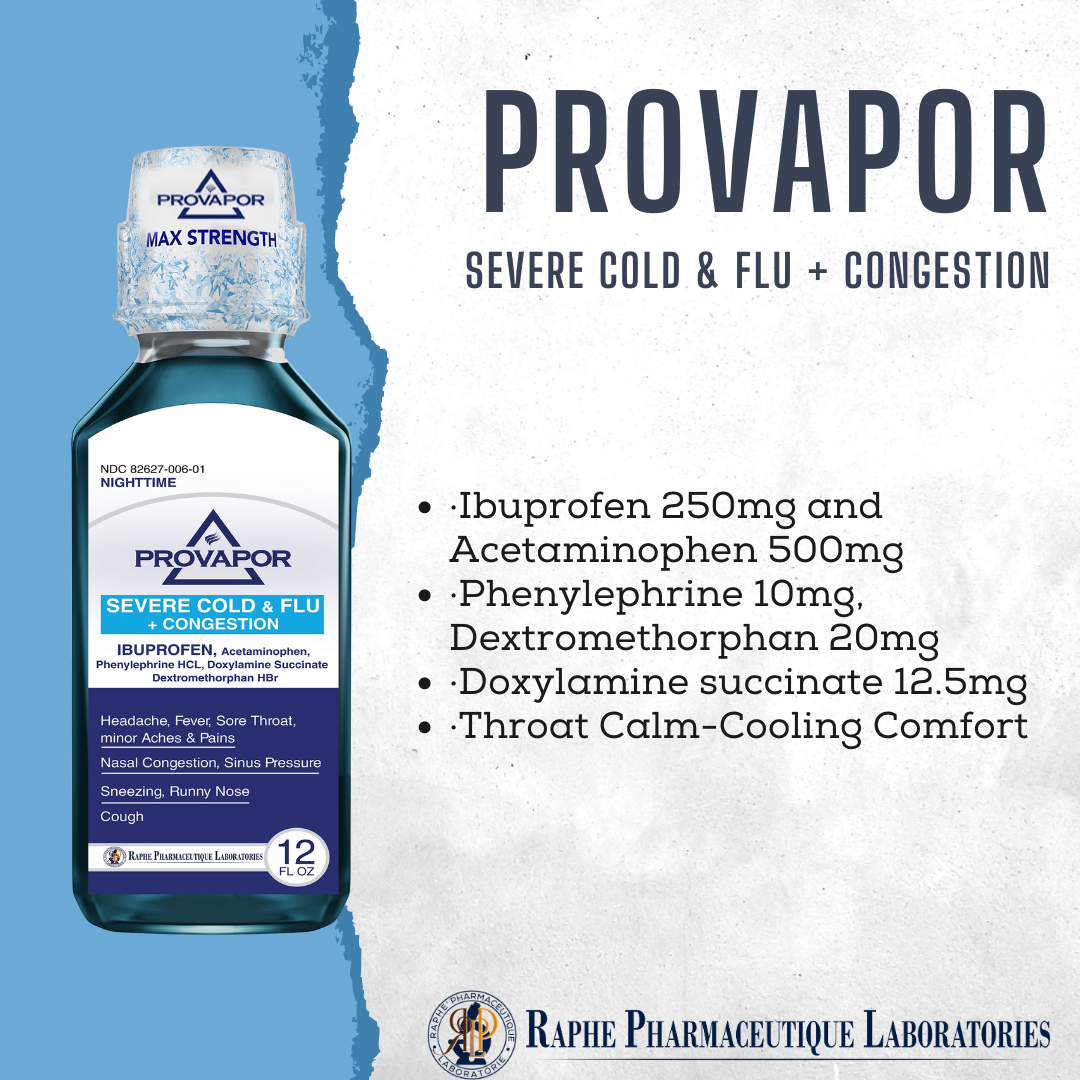
Multi-action Ibuprofen 250mg with Acetaminophen 500mg. Alleviating intra-sinus inflammation is a key factor in quick recovery from cold and flu. Mint flavor with 12 hours of cooling soothing comfort. Super great taste. No High-Fructose corn syrup. Ibuprofen, as a traditional NSAID, decreases the synthesis of pain- and inflammation, while Acetaminophen, a non-NSAID analgesic, exerts its effects on inhibiting and activating central serotonergic pathways. Working together, the two active ingredients provide 8 hours of tough pain relief and are superior to ibuprofen 250 mg and acetaminophen 650 mg.
The only COLD FLU syrup that fights pain using two different approaches to maximize efficacy while requiring lower maximum daily doses of each medicine.
- Only 20 ml per serving is required to achieve maximum relief compared to 30 ml of traditional cold flu syrup.
- Mint flavor with 12 hours of cooling soothing comfort.
- Super great taste. No High-Fructose corn syrup
- Ibuprofen 250mg, Acetaminophen 500mg, Phenylephrine 10mg, Dextromethorphan 20mg, Guaifenesin 400mg
- It provides faster relief from cold and flu symptoms, allowing patients to return to work and improve their health.
It is formulated to relieve patients by alleviating the inflammation of the bronchial cavity, nostrils, and sinuses, which is absent from the current products on the market.
Unit Price: Less than $2.99











Reviews
There are no reviews yet.